2024-3-26
2024-3-26 admin
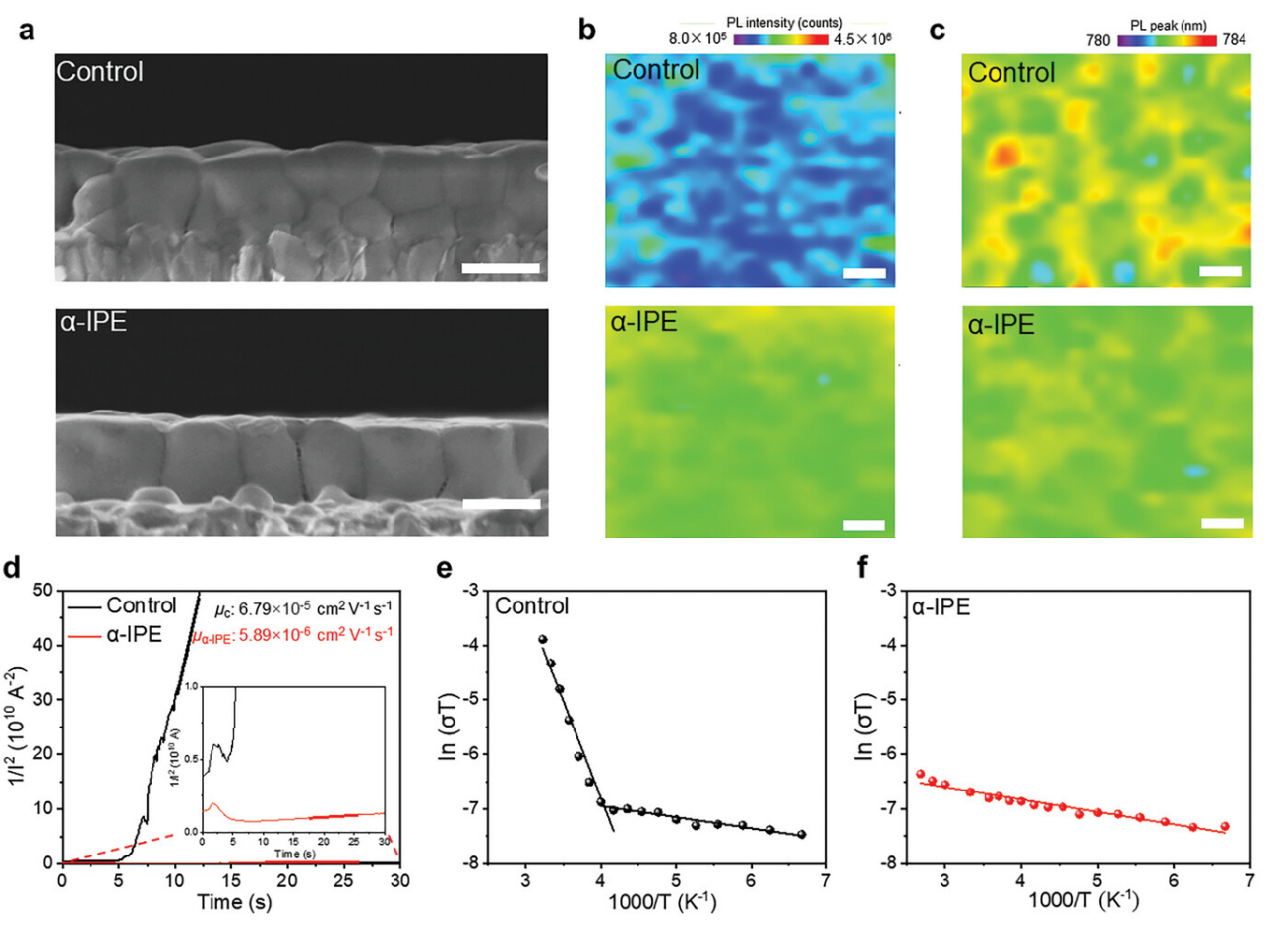
adminThe iodide vacancy defects generated during the perovskite crystallization process are a common issue that limits the efficiency and stability of perovskite solar cells (PSCs). Although excessive ionic iodides have been used to compensate for these vacancies, they are not effective in reducing defects through modulating the perovskite crystallization. Moreover, these iodide ions present in the perovskite films can act as interstitial defects, which are detrimental to the stability of the perovskite. Here, an effective approach to suppress the formation of vacancy defects by manipulating the coordination chemistry of lead polyhalides during perovskite crystallization is demonstrated. To achieve this suppression, an α-iodo ketone is introduced to undergo a process of Kornblum oxidation reaction that releases halide ions. This process induces a rapid collective transformation of lead polyhalides during the nucleation process and significantly reduces iodide vacancy defects. As a result, the ion mobility is decreased by one order of magnitude in perovskite film and the PSC achieves significantly improved thermal stability, maintaining 82% of its initial power conversion efficiency at 85 °C for 2800 h. These findings highlight the potential of halide ions released by the Kornblum oxidation reaction, which can be widely used for achieving high-performance perovskite optoelectronics.